A surprisingly good outreach platform has turned out to be Quora.com, a Q&A site where people ask questions and let anyone answer. So I will be replicating some of my answers from there to here. Enjoy…
Nature showed us how to do it, and it works great!
This is a nuclear waste repository, that held waste for 2 billion years.

Yes, you read that right: 2,000,000,000 years. That is 20,000 times more than what we consider to be adequate for a repository. And the only reason it is not longer than that is because…
a. that is how much time has passed since the waste was created
b. the waste has now decayed, completely. [1]
In the 1970’s, the Uranium ore find at Oklo, Gabon, Africa, gathered attention, because there was something “wrong” with the ore. It was as if the Uranium had already been used in a reactor.
As it turned out, it had indeed been in a reactor, a natural reactor. Billions of years back the isotope mix of Uranium was more like that we use in artificial reactors today. So all it needed was a bit of water to moderate the neutrons and — voilà! — nuclear fission, just like we do it today.
Nuclear fission means nuclear waste. These natural reactors also made waste. That meant a golden opportunity for us to examine what happened to the waste. The conclusion was astounding:
The waste stayed in place and moved less than 10 feet / 3 meters
This is despite the fact that the waste…
- was not packaged in fuel bundles
- was not encapsulated
- was subjected to violent temperature swings (these reactors worked in cycles of a few hours)
- was washed through by water for hundreds of thousands of years
The chief finding was that long-lived waste — the Transuraniums like Plutonium and Americium and other such Actinides — binds chemically to rock in a reducing environment and remains entirely immobile.
This is the key to why geological repositories work. Nature told us so. And that is why we are building repositories that way.
The Swedish KBS-3 method builds on the findings of Oklo and further research since the 1970’s. KBS-3 is already approved in Finland, and is in the process of being approved in Sweden.
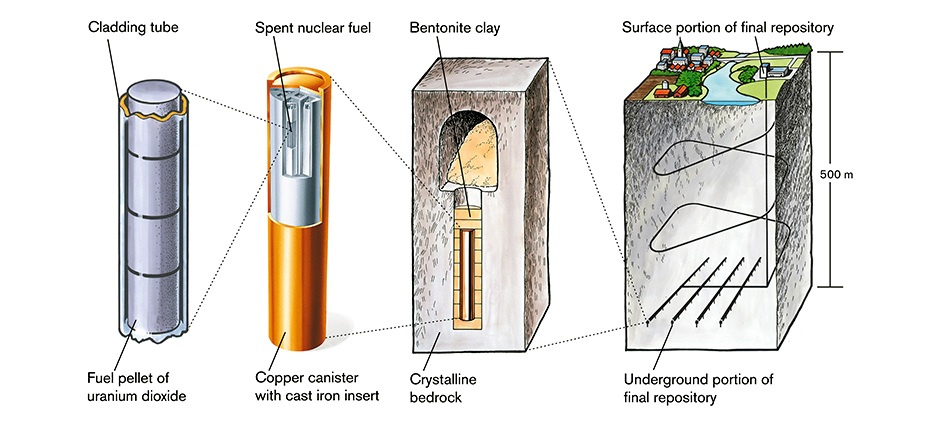
KBS-3 — besides using the reducing environment of the bedrock — also adds the following barriers.
- The fuel remains in the fuel rods, i.e. clad in Zirconium alloy. They are then placed in…
- Cast iron holders. The cast iron ensures rigidity, toughness, and that the environment will remain reducing even if water enters the…
- 2 inch / 50 mm thick corrosion resistant copper capsule that encapsulates the fuel bundles and their holder. That capsule is then surrounded by…
- A layer of water absorbent Bentonite clay. The clay acts as soft padding to keep the capsule from being subjected to movements of the bedrock. It is also meant to be wet, because when it wets it swells to a pressure of 50 atmospheres, and is pressed into all the cracks and fissures around…
- The bore hole, made 500 meters down into geologically stable bedrock, with a reducing environment and only small water movement.
The only thing that the Oklo reactors had was the reducing environment, and that alone held the waste in place for 2 billion years. KBS-3 will do the job.
So anyone that says there is no plan or no method or no site to deal with nuclear waste, is speaking — put in the plainest of the Queen’s English — complete and utter bollocks.
Footnotes
[1] The half-life of Plutonium-239 is: \[t_{1/2}= 24,100 y\]
So the tenth-life of Pu-239 is: \[t_{1/10} = t_{1/2} \left(\frac{ln(10)}{ln(2)}\right) \Rightarrow\]
\[t_{1/10} = 24,100 \cdot 3.32 \approx 80,000 y\]So 2 billion years makes for…
\[2,000,000,000 / 80,000 = 25,000\]…25,000 tenth-lives.
After about 110 or so tenth-lives, the original amount would have had to fill out the entirety of the known observable universe in order to have one atom left.
Leave a Comment